Circular No. 28/2012/TT-BKHCN is the foundational legal document stipulating the principles for implementing the declaration of standards conformity, technical regulations conformity, and the methods of conformity assessment with standards and technical regulations. This Circular serves as the basis for all other relevant ministries in Vietnam to develop regulatory documents concerning the principles of technical regulations certification and technical regulations declaration for products and goods under their management.

Overview of Circular No. 28/2012/TT-BKHCN
| Original number | 28/2012/TT-BKHCN |
| Amendment and supplement | 02/2017/TT-BKHCN |
| Issuing authority | Ministry of Science and Technology (MOST) |
| Issue date | 12/12/2012 |
| Take effect date | 27/01/2013 |
| Validity status | Still valid |
| Previous version | 24/2007/QĐ-BKHCN |
| Replacement version | N/A |
| Consolidate version number | 05/VBHN-BKHCN |

CEO ExtendMax - Trần Thanh Phương (Alex) - Leading expert in Conformity Certification and Conformity Declaration
The full text of Consolidated Circular No. 28/2012/TT-BKHCN
Below is the full text of the consolidated document of Circular No. 28/2012/TT-BKHCN and Circular No. 02/2017/TT-BKHCN. The consolidated document is numbered 05/VBHN-BKHCN, authenticated and consolidated on August 28, 2017.
Link to download original version from government website: https://chinhphu.vn/default.aspx?pageid=27160&docid=198238
| THE MINISTRY OF SCIENCE AND TECHNOLOGY | SOCIALIST REPUBLIC OF VIET NAM |
| No.: 05/VBHN-BKHCN | Hà Nội, August 28, 2017 |
PROVIDING FOR ANNOUNCEMENT OF STANDARD CONFORMITY AND ANNOUNCEMENT OF TECHNICAL-REGULATION CONFORMITY AND METHOD TO ASSESS CONFORMITY WITH STANDARDS AND TECHNICAL REGULATIONS
Circular No. 28/2012/TT-BKHCN dated December 12, 2012, issued by the Minister of Science and Technology, stipulating the declaration of standards conformity, technical regulations conformity, and methods of conformity assessment with standards and technical regulations, effective from January 27, 2013, as amended and supplemented by:
Circular No. 02/2017/TT-BKHCN dated March 31, 2017, issued by the Minister of Science and Technology, amending and supplementing certain articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, issued by the Minister of Science and Technology, stipulating the declaration of standards conformity, technical regulations conformity, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
Pursuant to the Law on Standards and Technical Regulations dated June 29, 2006;
Pursuant to the Law on product and goods quality dated November 21, 2007;
Pursuant to the Government’s Decree No.127/2007/ND-CP dated August 01, 2007, detailing implementation of a number of Articles of Law on Standards and Technical Regulations;
Pursuant to the Government’s Decree No.132/2008/ND-CP dated December 31, 2008, detailing implementation of a number of Articles of Law on product and goods quality;
Pursuant to the Government’s Decree No.28/2008/ND-CP dated March 14, 2008, defining the functions, tasks, powers and organizational structure of the Ministry of science and technology;
At the proposal of General Director of Directorate for Standards, Metrology and Quality;
The Minister of science and technology provides for announcement of standard conformity and announcement of technical-regulation conformity and method to assess the conformity with standards and technical regulations.
Chapter 1
Article 1. Scope of regulation
This Circular provides for announcement of standard conformity and announcement of technical-regulation conformity and method to assess the conformity with standards and technical regulations.
Article 2. Subjects of application
This Circular applies to organizations, individuals and management agencies related to assessment of the conformity, announcement of standard conformity and announcement of technical-regulation conformity.
Article 3. Interpretation of terms
In this Circular, the following terms are construed as follows:
1. Announcement of standard conformity means self-announcement of organizations and individuals that products, goods, services, process, environment are conformable to respective standards.
2. Announcement of technical-regulation conformity means self-announcement of organizations and individuals that products, goods, services, process, environment are conformable to respective national technical regulations.
3.2 The certification body conducting certification activities for standards conformity is an organization that has registered certification activities in accordance with the provisions of Decree No. 107/2016/ND-CP dated July 1, 2016, of the Government, stipulating the business conditions for conformity assessment services (hereinafter referred to as registered certification body).
4. The certification body conducting certification activities for technical regulations conformity is a certification body that has registered according to the provisions of Clause 3 of this Article and has been designated by a competent authority to conduct technical regulations conformity certification (hereinafter referred to as designated certification body).
5.3 The testing organization conducting product and goods quality testing activities is an organization that has registered testing activities in accordance with the provisions of Decree No. 107/2016/ND-CP dated July 1, 2016, of the Government, stipulating the business conditions for conformity assessment services.
6.4 Specialized agencies are professional agencies designated by the Ministry managing sectors, fields, or by the People's Committee of provinces and centrally-run cities, responsible for receiving the technical regulations conformity declaration dossiers of organizations and individuals.
Article 4. Standards Conformity Mark, Technical Regulations Conformity Mark
1. Standards Conformity Mark and its usage
The standards conformity mark is stipulated by the registered certification body in terms of shape, structure, manner of display, and usage for the certified object, and must meet the following basic requirements:
a) Ensure clarity, not causing confusion with other marks;
b) Fully display the symbol of the corresponding standard used as the basis for standards conformity certification.
In cases where organizations or individuals declare standards conformity based on self-assessment results, the shape, structure, and manner of display are not required, and the standards conformity mark cannot be used.
2. Technical Regulations Conformity Mark and its usage
a) The technical regulations conformity mark has the shape and size as specified in Appendix I of this Circular;
b) The technical regulations conformity mark is used directly on products, goods, or on packaging, technical documents, or labels attached to products, goods in a visible and readable position;
c) The technical regulations conformity mark must be indelible and not easily removable and reattached;
d) The technical regulations conformity mark can be enlarged or reduced but must maintain the correct proportion and basic dimensions of the technical regulations conformity mark specified in Appendix I of this Circular and be recognizable by the naked eye;
e) The technical regulations conformity mark must be designed and displayed in a single color, easily recognizable.
Article 5. Methods of Conformity Assessment
1. Conformity assessment is conducted according to one of the following methods:
a) Method 1: Typical sample testing;
b) Method 2: Typical sample testing and production process assessment; monitoring through sample testing taken from the market;
c) Method 3: Typical sample testing and production process assessment; monitoring through sample testing taken at the production site combined with production process assessment;
d) Method 4: Typical sample testing and production process assessment; monitoring through sample testing taken at the production site and from the market combined with production process assessment;
e) Method 5: Typical sample testing and production process assessment; monitoring through sample testing taken at the production site or from the market combined with production process assessment;
f) Method 6: Evaluation and monitoring of the management system;
g) Method 7: Testing and evaluating batches of products, goods;
h) Method 8: Testing or inspecting all products, goods.
2. The content, sequence, and principles of using the conformity assessment methods are specified in Appendix II of this Circular.
Article 6. Application of Conformity Assessment Methods
1. The conformity assessment method for standards is applied to each specific type of product, goods, service, process, or environment as chosen by the standards conformity certification organization or the organization or individual declaring standards conformity according to the conformity assessment methods stipulated in Article 5 of this Circular. The chosen conformity assessment method must be appropriate for the object being assessed to ensure the reliability of the conformity assessment results.
2. The conformity assessment method for technical regulations is applied to specific products, goods, services, processes, or environments as stipulated in the corresponding technical regulations.
3. The chosen conformity assessment method must be explicitly stated on the technical regulations conformity certificate.
Chapter II
DECLARATION OF STANDARDS CONFORMITY
Article 7. Principles of Standards Conformity Declaration
1. The objects of the standards conformity declaration are products, goods, services, processes, and environments specified in the corresponding standards. The declaration of standards conformity is a voluntary activity.
2. The declaration of conformity to the corresponding standards is based on:
a) The results of standards conformity certification conducted by a registered certification body; or
b) The results of self-assessment by the organization or individual declaring standards conformity.
The testing for conformity assessment must be conducted by a registered testing organization.
Article 8. Procedure for Standards Conformity Declaration
The declaration of standards conformity is carried out in the following steps:
1. Step 1: Assessing the conformity of the object of the standards conformity declaration with the corresponding standard (hereinafter referred to as standards conformity assessment).
a) The standards conformity assessment is conducted by a registered certification body (third party) or by the organization or individual declaring standards conformity (first party).
The standards conformity assessment is conducted according to the conformity assessment methods stipulated in Clause 1, Article 6 of this Circular;
b) The results of the standards conformity assessment stipulated in Point a, Clause 1 of this Article are the basis for the organization or individual to declare standards conformity.
2. Step 2: Registering the standards conformity declaration dossier at the Department of Standards, Metrology, and Quality of the province or centrally-run city where the organization or individual is registered for business or household business (hereinafter referred to as the Department).
Article 9. Dossier for Standards Conformity Declaration Registration
Organizations and individuals declaring standards conformity prepare 02 (two) sets of standards conformity declaration dossiers, including 01 (one) set submitted directly or sent by post to the Department and 01 (one) set kept at the organization or individual. The dossier components are as follows:
1. In case the standards conformity declaration is based on the results of standards conformity certification by a registered certification body (third party), the dossier for standards conformity declaration includes:
a) The standards conformity declaration (according to Form 2. CBHC/HQ stipulated in Appendix III of this Circular);
b) A certified copy of the documents proving the production and business activities of the organization or individual declaring standards conformity (Business Registration Certificate or Business License or Household Business Registration or Investment Certificate or Establishment Decision or other documents as prescribed by law);
c) A certified copy of the standard used as the basis for the declaration;
d) A certified copy of the standards conformity certificate issued by the registered certification body, along with the standards conformity mark sample.
During the dossier review process, if necessary, it will be cross-checked with the original or a certified copy will be required.
2. In case the standards conformity declaration is based on the results of self-assessment by the production and business organization or individual (first party), the dossier for standards conformity declaration includes:
a) The standards conformity declaration (according to Form 2. CBHC/HQ stipulated in Appendix III of this Circular);
b) A certified copy of the documents proving the production and business activities of the organization or individual declaring standards conformity (Business Registration Certificate or Business License or Household Business Registration or Investment Certificate or Establishment Decision or other documents as prescribed by law);
c) A certified copy of the standard used as the basis for the declaration;
d) If the organization or individual declaring standards conformity has not been granted a management system certification by a registered certification body (ISO 9001, ISO 22000, HACCP...), the dossier must include the production process along with the quality control plan developed and applied (according to Form 1. KHKSCL stipulated in Appendix III of this Circular) and the management system monitoring plan;
e) If the organization or individual declaring standards conformity has been granted a management system certification by a registered certification body (ISO 9001, ISO 22000, HACCP...), the dossier must include a certified copy of the valid management system certification;
f) A standards conformity assessment report (according to Form 5. BCĐG stipulated in Appendix III of this Circular) along with a certified copy of the test result certificate for samples within 12 months up to the time of submission of the standards conformity declaration dossier, issued by a registered testing organization.
During the dossier review process, if necessary, it will be cross-checked with the original or a certified copy will be required.
Article 10. Handling of Standards Conformity Declaration Dossiers
The standards conformity declaration dossiers sent to the Department are handled as follows:
1. For incomplete standards conformity declaration dossiers as stipulated in Article 9 of this Circular, within 03 (three) working days from the date of receipt of the standards conformity declaration dossier, the Department shall notify in writing the organization or individual declaring standards conformity to supplement the required documents as stipulated in Article 9 of this Circular. After 15 (fifteen) working days from the date the Department sends the written request, if the standards conformity declaration dossier is not fully supplemented according to regulations, the Department has the right to cancel the processing of this dossier.
2. For complete standards conformity declaration dossiers as stipulated in Article 9 of this Circular, within 05 (five) working days from the date of receipt of the standards conformity declaration dossier, the Department must organize an examination of the validity of the standards conformity declaration dossier to handle as follows:
a) In cases where the standards conformity declaration dossier is complete and valid, the Department shall issue a Notice of Receipt of the standards conformity declaration dossier to the organization or individual declaring standards conformity (according to Form 3. TBTNHS stipulated in Appendix III of this Circular). The Notice of Receipt of the standards conformity declaration dossier is valid for the same duration as the standards conformity certificate issued by the registered certification body or is valid for 03 (three) years from the date the organization's or individual's leader signs the standards conformity assessment report (for cases where the organization or individual self-assesses standards conformity).
b) In cases where the standards conformity declaration dossier is complete but invalid, the Department shall notify in writing the organization or individual declaring standards conformity of the reasons for not accepting the dossier.
Article 11. Responsibilities of Organizations and Individuals Declaring Standards Conformity
1. Select the conformity assessment method appropriate to the object of the standards conformity declaration to ensure the reliability of the assessment results.
2. Continuously maintain and be responsible for the conformity of the products, goods, services, processes, and environments registered for standards conformity declaration; maintain quality control, testing, and periodic monitoring at the production and business facilities of the organization or individual.
3. When discovering non-conformity of products, goods, services, processes, or environments that have been declared standards conformity during circulation or use, the organization or individual must:
a) Temporarily stop the release and recall non-conforming products and goods circulating in the market if the non-conforming products and goods pose a high risk of causing harm to users; stop the operation, exploitation of related processes, services, environments when necessary;
b) Implement corrective measures for the non-conformity;
c) Notify the Department in writing of the results of corrective measures before continuing to bring products, goods, services, processes, and environments into use, circulation, exploitation, or business.
4. Establish and maintain standards conformity declaration dossiers as follows:
a) In cases where the standards conformity declaration is based on the results of standards conformity certification by a registered certification body (third party), maintain the standards conformity declaration dossier including the original and copies of documents as stipulated in Clause 1, Article 9, and the monitoring evaluation dossier of the registered certification body;
b) In cases where the standards conformity declaration is based on self-assessment results by the production and business organization or individual (first party), maintain the standards conformity declaration dossier including the original and copies of documents as stipulated in Clause 2, Article 9, and the self-monitoring evaluation dossier of the organization or individual according to the monitoring plan.
5. Provide documents proving the conformity of products, goods, services, processes, and environments with the corresponding standards upon request by the competent state authority.
6. Provide a certified copy of the Notice of Receipt of the standards conformity declaration dossier to organizations or individuals doing business with products, goods, services, processes, and environments.
7. Re-declare when there are any changes to the content of the registered standards conformity declaration dossier or any changes to the features, uses, or characteristics of the products, goods, services that have been declared standards conformity.
Chapter III
DECLARATION OF TECHNICAL REGULATIONS CONFORMITY
Article 12. Principles of Technical Regulations Conformity Declaration 5
1. The objects of the technical regulations conformity declaration are products, goods, services, processes, and environments specified in the national technical regulations issued by the sectoral management ministries or in the local technical regulations issued by the People's Committees of provinces and centrally-run cities. The declaration of technical regulations conformity is a mandatory activity.
2. The sectoral management ministries identify group 2 products and goods, develop the corresponding national technical regulations, and management measures for the technical regulations conformity declaration as stipulated in the national technical regulations after obtaining consensus from the Ministry of Science and Technology.
3. The technical regulations conformity declaration activity for imported goods stipulated in this Circular is conducted by organizations and individuals performing state inspection on the quality of imported goods.
4. The technical regulations conformity declaration is based on one of the following measures:
a) Results of self-assessment of conformity by organizations or individuals (hereinafter referred to as self-assessment results);
b) Certification results from a registered or legally recognized certification body;
c) Certification results from a designated certification body.
The testing for technical regulations conformity declaration is conducted at a registered or legally recognized testing organization.
In cases where the conformity assessment results of a foreign conformity assessment body are used, the foreign conformity assessment body must be legally recognized or designated by the competent state management agency.
5. In cases where products and goods are managed by multiple technical regulations, organizations and individuals must register the technical regulations conformity declaration with the corresponding specialized agencies, and the technical regulations conformity mark can only be used when the products and goods have fully implemented the management measures as stipulated in the corresponding technical regulations.
Article 13. Procedure for Technical Regulations Conformity Declaration 6
1. In cases where the technical regulations conformity declaration is based on self-assessment results:
a) For domestically produced products and goods:
- Organizations and individuals submit the technical regulations conformity declaration dossier as stipulated in Article 14 of this Circular to the specialized agency for declaration based on their self-assessment results;
- After submitting the technical regulations conformity declaration dossier to the specialized agency, organizations and individuals are allowed to circulate the goods.
b) For imported goods:
- Organizations and individuals register for quality inspection of imported goods with the following information: name of the importing organization or individual, address, phone, fax; name of goods, brand, model; technical characteristics; origin, manufacturer; weight, quantity; port of entry; import time; contract; packing list; invoice; bill of lading; customs declaration of imported goods; technical regulation number; quality commitment that the product or goods conform to the technical regulations and declared standards, and take full legal responsibility for the quality of the products and goods.
Within 01 working day, the specialized agency confirms that the organization or individual has registered for quality inspection of imported goods on the registration form of the organization or individual;
- Organizations and individuals submit the registration form with the confirmation of the specialized agency to the customs authority to clear the goods;
- Within 15 working days from the date of goods clearance, organizations and individuals must submit the self-assessment results as stipulated in point b, Clause 1, Article 14 of this Circular to the specialized agency.
Organizations and individuals are fully responsible for the self-assessment results and ensure that the goods conform to the technical regulations and declared standards. In cases where goods do not conform to the technical regulations and declared standards, organizations and individuals must promptly report to the specialized agency and organize the handling and recall of these goods according to legal regulations.
2. In cases where the technical regulations conformity declaration is based on the assessment results of a registered or recognized certification body (hereinafter referred to as the certification body):
a) For domestically produced products and goods:
- Organizations and individuals submit the technical regulations conformity declaration dossier as stipulated in Article 14 of this Circular to the specialized agency for declaration based on the assessment results of the certification body;
- After receiving the Notice of Receipt of the technical regulations conformity declaration dossier from the specialized agency, organizations and individuals are allowed to circulate the goods.
b) For imported goods:
- Organizations and individuals register for quality inspection of imported goods with the following information: name of the importing organization or individual, address, phone, fax; name of goods, brand, model; technical characteristics; origin, manufacturer; weight, quantity; port of entry; import time; contract; packing list; invoice; bill of lading; customs declaration of imported goods; technical regulation number; quality commitment that the product or goods conform to the technical regulations and declared standards, and take full legal responsibility for the quality of the products and goods.
Within 01 working day, the specialized agency confirms that the organization or individual has registered for quality inspection of imported goods on the registration form of the organization or individual;
- Organizations and individuals submit the registration form with the confirmation of the specialized agency to the customs authority to clear the goods;
- Within 15 working days from the date of goods clearance, organizations and individuals must submit a certified copy of the Technical Regulations Conformity Certificate to the specialized agency.
In cases where the goods have been assessed by the certification body in the exporting country, within 03 working days from the date of goods clearance, organizations and individuals must submit a certified copy of the Technical Regulations Conformity Certificate to the specialized agency.
Organizations and individuals are fully responsible and ensure that the goods conform to the technical regulations and declared standards. In cases where goods do not conform to the technical regulations and declared standards, organizations and individuals must promptly report to the specialized agency and organize the handling and recall of these goods according to legal regulations.
3. In cases where the technical regulations conformity declaration is based on the assessment results of a designated certification body:
a) For domestically produced products and goods:
- Organizations and individuals submit the technical regulations conformity declaration dossier as stipulated in Article 14 of this Circular, along with a certified copy of the Technical Regulations Conformity Certificate issued by the designated certification body to the specialized agency to receive the Notice of Receipt of the technical regulations conformity declaration dossier;
- After receiving the Notice of Receipt of the technical regulations conformity declaration dossier, organizations and individuals are allowed to circulate the goods.
b) For imported goods:
- Organizations and individuals register for quality inspection of imported goods with the following information: name of the importing organization or individual, address, phone, fax; name of goods, brand, model; technical characteristics; origin, manufacturer; weight, quantity; port of entry; import time; contract; packing list; invoice; bill of lading; customs declaration of imported goods; technical regulation number; quality commitment that the product or goods conform to the technical regulations and declared standards, and take full legal responsibility for the quality of the products and goods, along with a certified copy of the Technical Regulations Conformity Certificate issued by the designated certification body;
- The specialized agency issues the Notice of Results of State Inspection on the Quality of Imported Goods;
- After receiving the Notice of Results of State Inspection on the Quality of Imported Goods, organizations and individuals submit a certified copy of this Notice to the customs authority to clear the goods.
4. The specialized agency stipulated in Clauses 1, 2, and 3 of this Article shall handle the technical regulations conformity declaration dossier according to the provisions of Article 15 of this Circular.
Article 14. Technical Regulations Conformity Declaration Dossier 7
Organizations and individuals declaring technical regulations conformity shall prepare the technical regulations conformity declaration dossier and submit it directly or send it by post to the specialized agency designated and authorized by the Ministry managing sectors, fields, or the People's Committees of provinces and centrally-run cities. The dossier components are stipulated as follows:
1. In cases where the technical regulations conformity declaration is based on the self-assessment results of organizations or individuals (first party), the technical regulations conformity declaration dossier includes:
a) The technical regulations conformity declaration (according to Form 2. CBHC/HQ stipulated in Appendix III of this Circular);
b) The self-assessment report including the following information:
-
Name of the organization or individual; address; phone, fax;
-
Name of the product or goods;
-
Technical regulation number;
-
Conclusion that the product or goods conform to the technical regulations;
-
Commitment that the quality of the product or goods conforms to the technical regulations and declared standards, and full legal responsibility for the quality of the product or goods and the self-assessment results.
The self-assessment report is based on the results of the organization's or individual's self-implementation or based on the assessment results of a registered conformity assessment body.
2. In cases where the technical regulations conformity declaration is based on the certification results of a registered certification body or a designated certification body (third party), the technical regulations conformity declaration dossier includes:
a) The technical regulations conformity declaration (according to Form 2. CBHC/HQ stipulated in Appendix III of this Circular);
b) A certified copy of the corresponding Technical Regulations Conformity Certificate along with the technical regulations conformity mark sample issued by the registered certification body or the designated certification body to the organization or individual.
Article 15. Handling of Technical Regulations Conformity Declaration Dossiers
The technical regulations conformity declaration dossiers submitted to the specialized agency are handled as follows:
1. For incomplete technical regulations conformity declaration dossiers as stipulated in Article 14 of this Circular, within 03 (three) working days from the date of receipt of the technical regulations conformity declaration dossier, the specialized agency shall notify in writing the organization or individual declaring technical regulations conformity to supplement the required documents according to regulations. After 15 (fifteen) working days from the date the specialized agency sends the written request, if the technical regulations conformity declaration dossier is not fully supplemented according to regulations, the specialized agency has the right to cancel the processing of this dossier.
2. For complete technical regulations conformity declaration dossiers as stipulated in Article 14 of this Circular, within 05 (five) working days from the date of receipt of the technical regulations conformity declaration dossier, the specialized agency shall organize an examination of the validity of the technical regulations conformity declaration dossier:
a) In cases where the technical regulations conformity declaration dossier is complete and valid, the specialized agency shall issue a Notice of Receipt of the technical regulations conformity declaration dossier to the organization or individual declaring technical regulations conformity (according to Form 3. TBTNHS stipulated in Appendix III of this Circular).
The Notice of Receipt of the technical regulations conformity declaration dossier is valid for the same duration as the Technical Regulations Conformity Certificate issued by the designated certification body or is valid for three (03) years from the date the organization's or individual's leader signs the technical regulations conformity assessment report (for cases where the organization or individual self-assesses technical regulations conformity);
b) In cases where the technical regulations conformity declaration dossier is complete but invalid, the specialized agency shall notify in writing the organization or individual declaring technical regulations conformity of the reasons for not accepting the dossier.
Article 16. Responsibilities of Organizations and Individuals Declaring Technical Regulations Conformity
1. Announce on appropriate media about their technical regulations conformity declaration to ensure that users of the products or goods can easily access the information.
2. Continuously maintain and be responsible for the conformity of the products, goods, services, processes, and environments that have been declared technical regulations conformity; maintain quality control, testing, and periodic monitoring.
3. Use the technical regulations conformity mark for products and goods that have been declared technical regulations conformity according to Clause 2, Article 4 of this Circular before releasing them into the market. Maintain a logbook and report the use of the technical regulations conformity mark to the designated certification body annually.
4. When discovering non-conformity of products, goods, services, processes, or environments that have been declared technical regulations conformity during circulation or use, the organization or individual must:
a) Promptly notify the specialized agency in writing about the non-conformity;
b) Temporarily stop the release and recall non-conforming products and goods circulating in the market if the non-conforming products and goods pose a high risk of causing harm to users; stop the operation and exploitation of related processes, services, and environments when necessary;
c) Implement corrective measures for the non-conformity;
d) Notify the specialized agency in writing about the results of the corrective measures before continuing to bring the products, goods, services, processes, and environments into use, circulation, exploitation, or business.
5. Establish and maintain the technical regulations conformity declaration dossier as the basis for inspection and examination by state management agencies as follows:
a) In cases where the technical regulations conformity declaration is based on the certification results of a designated certification body (third party), maintain the technical regulations conformity declaration dossier including the originals and copies of documents as stipulated in Clause 1, Article 14, and the monitoring evaluation dossier of the designated certification body;
b) In cases where the technical regulations conformity declaration is based on self-assessment results by the production and business organization or individual (first party), maintain the technical regulations conformity declaration dossier including the originals and copies of documents as stipulated in Clause 2, Article 14, and the self-monitoring evaluation dossier of the organization or individual according to the monitoring plan.
6. Provide documents proving the conformity of products, goods, services, processes, and environments with the corresponding technical regulations upon request by the competent state authority.
7. Provide certified copies of the corresponding documents stipulated in Clauses 2 and 3, Article 13 of this Circular (Notice of Receipt of the technical regulations conformity declaration dossier or Technical Regulations Conformity Certificate or Notice of Results of State Inspection on the Quality of Imported Goods) to organizations or individuals doing business with products, goods, services, processes, and environments; or use appropriate measures to ensure that organizations or individuals doing business with products, goods, services can trace the origin and information about the products, goods, and services conforming to the declared standards and technical regulations.
8. Re-declare when there are any changes to the content of the registered technical regulations conformity declaration dossier or any changes to the features, uses, or characteristics of the products, goods, and services that have been declared technical regulations conformity.
Chapter IV
IMPLEMENTATION ORGANIZATION
Article 17. Responsibilities of Management Agencies
1. Responsibilities of sectoral management ministries and the People's Committees of provinces and centrally-run cities:
a) Direct the technical regulations conformity declaration activities according to the provisions of this Circular when issuing corresponding technical regulations for management;
b) Designate a focal agency responsible for managing technical regulations conformity declaration activities in the assigned field; notify the list of focal agencies to relevant organizations and individuals for implementation and send it to the Ministry of Science and Technology for coordination and management;
c) Assign responsibilities to specialized agencies for receiving technical regulations conformity declaration dossiers;
d) Periodically in December each year, or unexpectedly when required, compile and report the situation of receiving technical regulations conformity declaration dossiers (according to Form 4. BCTNHS stipulated in Appendix III of Circular No. 28/2012/TT-BKHCN) to the Ministry of Science and Technology for consolidation and reporting to the Prime Minister.
e) Lead and coordinate with the Ministry of Science and Technology to identify group 2 products and goods, national technical regulations, and select appropriate management measures according to the technical regulations conformity declaration principles stipulated in Clause 2, Article 1 of this Circular.
2. Responsibilities of the Directorate for Standards, Metrology, and Quality as the focal agency designated according to Point b, Clause 1 of this Article:
a) Assist the Ministry of Science and Technology in uniformly managing and guiding conformity assessment, standards conformity declaration, and technical regulations conformity declaration activities. Coordinate with sectoral management ministries to identify group 2 products and goods, national technical regulations, and select appropriate management measures managed by the sectoral management ministries according to the technical regulations conformity declaration principles stipulated in Clause 2, Article 1 of this Circular;
b) Coordinate with the central focal agencies of sectoral management ministries, the Departments of Science and Technology of provinces, and centrally-run cities in urging and guiding the implementation of standards conformity declaration and technical regulations conformity declaration according to the provisions of this Circular;
c) Monitor the standards conformity declaration and technical regulations conformity declaration situation for products, goods, services, processes, and environments under the management responsibility of the Ministry of Science and Technology based on reports from the local Departments of Standards, Metrology, and Quality; monitor the designation of conformity assessment activities by sectoral management ministries.
3. Responsibilities of the focal agencies designated according to Point b, Clause 1 of this Article under sectoral management ministries and the People's Committees of provinces and centrally-run cities:
a) Monitor and manage the technical regulations conformity declaration registration activities of specialized agencies; coordinate with the Directorate for Standards, Metrology, and Quality in managing technical regulations conformity declaration activities; annually compile reports and send them to sectoral management ministries and the People's Committees of provinces and centrally-run cities regarding the designation of conformity assessment bodies, and send them to the Directorate for Standards, Metrology, and Quality for coordination and management;
b) Compile the situation of receiving technical regulations conformity declaration dossiers of specialized agencies and periodically in December each year, or unexpectedly when required, report to sectoral management ministries and the People's Committees of provinces and centrally-run cities.
4. Responsibilities of specialized agencies designated by sectoral management ministries and the People's Committees of provinces and centrally-run cities:
a) Receive registration and manage technical regulations conformity declaration dossiers; cancel or suspend the results of receiving technical regulations conformity declaration dossiers for products, goods, services, processes, and environments managed by national technical regulations issued by sectoral management ministries and related local technical regulations;
b) Regularly update the status of receiving technical regulations conformity declaration dossiers and publicly announce it on their website with the following contents:
- Name of the organization or individual declaring technical regulations conformity;
- Products or goods declaring technical regulations conformity;
- Technical regulation number;
- Type of assessment: First party (name of the organization or individual) or third party (name of the certification body or designated certification body).
c) Coordinate with local Departments of Standards, Metrology, and Quality in providing information about technical regulations conformity declaration to facilitate quality inspection of products and goods;
d) Periodically in December each year, or unexpectedly when required, compile and report to the focal agency the list of products, goods, services, processes, and environments and the situation of receiving technical regulations conformity declaration dossiers (according to Form 4. BCTNHS stipulated in Appendix III of Circular No. 28/2012/TT-BKHCN).
5. Responsibilities of the local Departments of Standards, Metrology, and Quality under the Departments of Science and Technology of provinces and centrally-run cities:
a) Receive registration and manage standards conformity declaration dossiers; cancel or suspend the results of receiving standards conformity declaration dossiers of production and business organizations and individuals in the locality and publicly announce it on the website of the Department of Science and Technology or the local Department of Standards, Metrology, and Quality;
b) Receive registration and manage technical regulations conformity declaration dossiers; cancel or suspend the results of receiving technical regulations conformity declaration dossiers for products, goods, services, processes, and environments managed by national technical regulations issued by the Ministry of Science and Technology and related local technical regulations; regularly update the status of receiving technical regulations conformity declaration dossiers and publicly announce it on the website of the Department of Science and Technology or the local Department of Standards, Metrology, and Quality with the following contents:
- Name of the organization or individual declaring technical regulations conformity;
- Products or goods declaring technical regulations conformity;
- Technical regulation number;
- Type of assessment: First party (name of the organization or individual) or third party (name of the certification body or designated certification body).
c) Coordinate with local specialized agencies in providing information about standards conformity declaration to facilitate quality inspection of products and goods;
d) Periodically in December each year, or unexpectedly when required, compile and report to the Directorate for Standards, Metrology, and Quality the situation of receiving standards conformity declaration and technical regulations conformity declaration dossiers (according to Form 4. BCTNHS stipulated in Appendix III of Circular No. 28/2012/TT-BKHCN) according to the provisions of Points a and b of this Clause.
Article 18. Inspection, Examination, and Handling of Violations
1. Competent state management agencies shall conduct inspections, examinations, and handle legal violations in the activities of standards conformity declaration and technical regulations conformity declaration according to the provisions of this Circular and other relevant current regulations.
2. Organizations and individuals violating the regulations on standards conformity declaration and technical regulations conformity declaration, depending on the nature and extent of the violation, shall be handled according to the relevant current legal provisions.
Article 19. Implementation Provisions 17
This Circular takes effect from January 27, 2013, and replaces Decision No. 24/2007/QD-BKHCN dated September 28, 2007, of the Minister of Science and Technology on the issuance of regulations on standards conformity certification, technical regulations conformity certification, standards conformity declaration, and technical regulations conformity declaration.
Article 20. Implementation Organization
1. Ministers, heads of ministerial-level agencies, and chairpersons of the People's Committees of provinces and centrally-run cities are responsible for organizing the implementation of this Circular.
2. The Director General of the Directorate for Standards, Metrology, and Quality is responsible for guiding and organizing the implementation of this Circular.
3. During the implementation process, if any issues or difficulties arise, organizations and individuals shall promptly report them in writing to the Ministry of Science and Technology for study and amendment./.
|
Recipients:
|
CERTIFIED CONSOLIDATED DOCUMENT On behalf of the Minister Deputy Minister
Trần Văn Tùng
|
ANNEX I
SHAPE AND SIZE OF THE TECHNICAL REGULATIONS CONFORMITY MARK
(Issued together with Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology)
SHAPE AND SIZE OF THE TECHNICAL REGULATIONS CONFORMITY MARK
1. The technical regulations conformity mark has the shape described in Figure 1.

Hình 1. Hình dạng của dấu hợp quy
2. The technical regulations conformity mark has the size described in Figure 2.
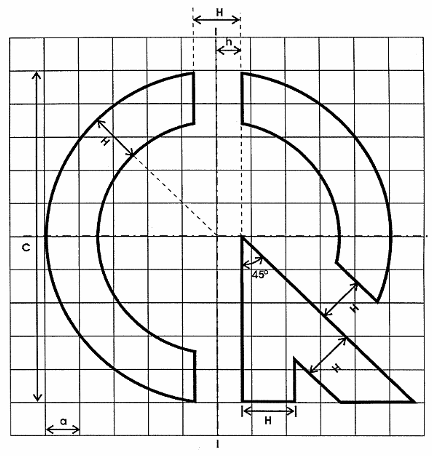
Hình 2. Kích thước cơ bản của dấu hợp quy
Note:
H = 1,5 a
h = 0,5 H
C = 7,5 H
ANNEX II
CONTENT, SEQUENCE, AND PRINCIPLES OF USING CONFORMITY ASSESSMENT METHODS
(Issued together with Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology)
CONTENT, SEQUENCE, AND PRINCIPLES OF USING CONFORMITY ASSESSMENT METHODS
I. Method 1: Typical Sample Testing
Method 1 involves testing a typical sample of the product or goods to conclude its conformity. The conformity conclusion is valid for the type and model of the product or goods that have been sampled and tested.
1. The content and sequence of the main activities in Method 1 include:
1.1. Sampling:
Take a typical sample of the product or goods. A typical sample is representative of a specific type and model of the product or goods produced under the same design, conditions, and using the same materials.
The number of samples must be sufficient for testing and retention.
1.2. Assessing the conformity of the sample:
The product or goods sample is tested at a laboratory registered in accordance with the law, which may include the manufacturer's laboratory. Priority is given to designated and accredited laboratories.
The characteristics of the product or goods to be tested and the testing methods are specified in the corresponding standards and technical regulations.
1.3. Processing the conformity assessment results:
Compare the characteristics of the product or goods based on the sample test results with the requirements of the corresponding standards and technical regulations.
1.4. Concluding conformity:
Conclude the conformity of the product or goods with the requirements of the corresponding standards and technical regulations. The product or goods is considered conforming if all the test sample indicators meet the specified levels of the standards and technical regulations.
2. Principles for using Method 1:
Method 1 is used to assess the conformity of products or goods under the following conditions:
a) The design of the product or goods allows for clear identification by specific type and model;
b) It is not possible to review the requirements to ensure stable quality maintenance.
II. Method 2: Typical Sample Testing and Production Process Assessment; Monitoring through Market Sample Testing
Method 2 is based on the results of typical sample testing and production process assessment to conclude the conformity of the product or goods. Subsequent monitoring is conducted through testing samples taken from the market.
1. The content and sequence of the main activities in Method 2 include:
1.1. Sampling:
Proceed as specified in item 1.1 of Method 1.
1.2. Assessing the conformity of the sample:
Proceed as specified in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
The production process assessment must fully consider the manufacturer's control conditions related to the product formation to ensure stable quality maintenance. Control conditions include:
a) Control of the technical documentation of the product (design documents, technical standards of the product);
b) Control of the entire production process from input, through intermediate stages, to product formation, including packaging, handling, storage, and transportation;
c) Control of the quality of raw materials, semi-finished products, and finished products;
d) Control of technological equipment and measuring, testing, and inspection equipment;
e) Control of the skill levels of workers and technical staff;
f) Other necessary technical contents.
If the manufacturer has a quality management system certificate from a certification body registered or recognized for the relevant production field, production process assessment is not required. However, if there is evidence of the ineffectiveness of the quality management system, the certification body should assess the production process and report to the Directorate for Standards, Metrology, and Quality.
1.4. Processing the conformity assessment results:
Compare the characteristics of the product or goods based on the sample test results with the requirements of the corresponding standards and technical regulations.
Review the conformity of the production process with the requirements specified in item 1.3 of this method.
1.5. Concluding conformity:
Conclude the conformity of the product or goods with the requirements of the corresponding standards and technical regulations. The product or goods is considered conforming if it meets the following two conditions:
a) All indicators of the test sample meet the specified levels of the standards and technical regulations;
b) The production process assessment results meet the requirements.
The conformity conclusion is valid for a maximum of 3 years, provided the product or goods is monitored.
1.6. Monitoring:
During the validity period of the conformity conclusion, the product or goods must be monitored through market sample testing. The monitoring frequency must not exceed once every 12 months.
Product or goods sample testing is conducted as specified in items 1.1, 1.2, and 1.3 of Method 1.
Monitoring assessment results are used as the basis for deciding whether to maintain, suspend, or cancel the conformity conclusion.
2. Principles for using Method 2:
Method 2 is used to assess the conformity of products or goods under the following conditions:
a) Products or goods have low-risk potential for safety, health, and environment;
b) The design of the product or goods allows for clear identification by specific type and model;
c) It is important to maintain stable quality characteristics during production;
d) The quality of the product or goods may change during market distribution;
e) The organization or individual producing or trading the product or goods has effective measures to recall the product or goods from the market if non-conformity is detected during monitoring.
III. Method 3: Typical Sample Testing and Production Process Assessment; Monitoring through Sample Testing at the Production Site Combined with Production Process Assessment
Method 3 is based on the results of typical sample testing and production process assessment to conclude the conformity of the product or goods. The monitoring assessment is conducted through sample testing at the production site combined with production process assessment.
1. The content and sequence of the main activities in Method 3 include:
1.1. Sampling:
Proceed as specified in item 1.1 of Method 1.
1.2. Assessing the conformity of the sample:
Proceed as specified in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
Proceed as specified in item 1.3 of Method 2.
1.4. Processing the conformity assessment results:
Proceed as specified in item 1.4 of Method 2.
1.5. Concluding conformity:
Proceed as specified in item 1.5 of Method 2.
1.6. Monitoring:
During the validity period of the conformity conclusion, the product or goods must be monitored through sample testing at the production site combined with production process assessment. The monitoring frequency must not exceed once every 12 months.
Product or goods sample testing is conducted as specified in items 1.1, 1.2, and 1.3 of Method 1.
The production process assessment is conducted as specified in item 1.3 of Method 2.
Monitoring assessment results are used as the basis for deciding whether to maintain, suspend, or cancel the conformity conclusion.
2. Principles for using Method 3:
Method 3 is used to assess the conformity of products or goods under the following conditions:
a) Products or goods pose a higher risk to safety, health, and the environment compared to those assessed using Method 2;
b) The design of the product or goods allows for clear identification by specific type and model;
c) It is important to maintain stable quality characteristics during production;
d) The quality of the product or goods is inherently less likely or unlikely to change during market distribution;
e) Effective measures to recall the product or goods from the market are difficult to implement if non-conformity is detected during monitoring.
IV. Method 4: Typical Sample Testing and Production Process Assessment; Monitoring through Sample Testing at the Production Site and Market Combined with Production Process Assessment
Method 4 is based on the results of typical sample testing and production process assessment to conclude the conformity of the product or goods. Subsequent monitoring is conducted through sample testing at the production site and market combined with production process assessment.
1. The content and sequence of the main activities in Method 4 include:
1.1. Sampling:
Proceed as specified in item 1.1 of Method 1.
1.2. Assessing the conformity of the sample:
Proceed as specified in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
Proceed as specified in item 1.3 of Method 2.
1.4. Processing the conformity assessment results:
Proceed as specified in item 1.4 of Method 2.
1.5. Concluding conformity:
Proceed as specified in item 1.5 of Method 2.
1.6. Monitoring:
During the validity period of the conformity notice, the product or goods must be monitored through sample testing at the production site and market combined with production process assessment. The monitoring frequency must not exceed once every 12 months.
Product or goods sample testing is conducted as specified in items 1.1, 1.2, and 1.3 of Method 1.
The production process assessment is conducted as specified in item 1.3 of Method 2.
Monitoring assessment results are used as the basis for deciding whether to maintain, suspend, or cancel the conformity conclusion.
2. Principles for using Method 4:
Method 4 is used to assess the conformity of products or goods under the following conditions:
a) Products or goods pose a higher risk to safety, health, and the environment compared to those assessed using Method 3;
b) The design of the product or goods allows for clear identification by specific type and model;
c) It is important to maintain stable quality characteristics during production;
d) The quality of the product or goods is likely to become unstable during production and change during market distribution;
e) Measures allow for the recall of the product or goods from the market if non-conformity is detected during monitoring.
V. Method 5: Typical Sample Testing and Production Process Assessment; Monitoring through Sample Testing at the Production Site or Market Combined with Production Process Assessment
Method 5 is based on the results of typical sample testing and production process assessment to conclude the conformity of the product or goods. The monitoring assessment is conducted through sample testing at the production site or market combined with production process assessment.
1. The content and sequence of the main activities in Method 5 include:
1.1. Sampling:
Proceed as specified in item 1.1 of Method 1.
1.2. Assessing the conformity of the sample:
Proceed as specified in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
Proceed as specified in item 1.3 of Method 2.
1.4. Processing the conformity assessment results:
Proceed as specified in item 1.4 of Method 2.
1.5. Concluding conformity:
Proceed as specified in item 1.5 of Method 2.
1.6. Monitoring:
During the validity period of the conformity conclusion, the product or goods must be monitored through sample testing at the production site or market combined with production process assessment. The monitoring frequency must not exceed once every 12 months.
Product or goods sample testing is conducted as specified in items 1.1, 1.2, and 1.3 of Method 1.
The production process assessment is conducted as specified in item 1.3 of Method 2.
Monitoring assessment results are used as the basis for deciding whether to maintain, suspend, or cancel the conformity conclusion.
2. Principles for using Method 5:
Method 5 is used to assess the conformity of products or goods under the following conditions:
a) A high-reliability method like Method 4 is required, but flexibility in monitoring measures is allowed to reduce costs;
b) A commonly applied method is required to facilitate mutual recognition of conformity assessment results.
VI. Method 6: Evaluation and Monitoring of the Management System
Method 6 is based on the evaluation of the management system to conclude the conformity of the management system with the requirements of the corresponding standards and technical regulations.
1. The content and sequence of the main activities in Method 6 include:
1.1. Assessing the conformity of the management system:
-
The management system is evaluated according to the requirements of the corresponding standards and technical regulations.
-
The evaluation report is compared with the requirements of the corresponding standards and technical regulations.
1.2. Concluding conformity:
Based on the evaluation report, conclude the conformity of the management system with the requirements of the corresponding standards and technical regulations.
The conformity conclusion of the management system is valid for a maximum of 3 years, provided the management system is monitored.
1.3. Monitoring the management system:
-
Monitoring is conducted through the evaluation of the management system, with a monitoring frequency not exceeding once every 12 months.
-
Monitoring results are used as the basis for deciding whether to continue maintaining, suspending, or canceling the conformity of the management system.
2. Principles for using Method 6:
Method 6 is used to assess the conformity of processes, services, and environments with a management system according to the requirements of the corresponding standards and technical regulations.
VII. Method 7: Testing and Evaluating a Batch of Products or Goods
Method 7 is based on the results of testing samples of products or goods taken using statistical sampling methods for the batch to conclude the conformity of the batch. The conformity conclusion is valid only for the specific batch of products or goods and does not require further monitoring measures.
1. The content and sequence of the main activities in Method 7 include:
1.1. Sampling:
Samples are taken using statistical sampling methods to ensure they are representative of the entire batch.
The number of samples must be sufficient for testing and retention.
1.2. Assessing the conformity of the sample:
Proceed as specified in item 1.2 of Method 1.
1.3. Processing the conformity assessment results:
Compare the characteristics of the product or goods based on the sample test results with the requirements of the corresponding standards and technical regulations.
1.4. Concluding conformity:
The batch of products or goods is considered conforming if the number of non-conforming sample results is within the allowable limits.
The batch of products or goods is considered non-conforming if the number of non-conforming sample results exceeds the allowable limits.
2. Principles for using Method 7:
Method 7 is used to assess the conformity of products or goods under the following conditions:
a) Products or goods are homogeneous and can be defined in batches;
b) It is not possible to review the requirements to ensure stable quality maintenance.
VIII. Method 8: Testing or Inspecting All Products or Goods
Method 8 is based on the results of testing or inspecting all products or goods to conclude conformity before they are circulated or used. The conformity conclusion is valid only for each individual product or goods and does not require further monitoring measures.
1. The content and sequence of the main activities in Method 8 include:
**1.1. Identifying products or goods to be tested or inspected;
1.2. Assessing the conformity of the products or goods:
a) Testing or inspecting the products or goods is conducted by laboratories or inspection bodies registered in the relevant field, capable of conducting tests at the production site, installation site, usage site, or at the laboratory or inspection body.
Priority is given to accredited laboratories or inspection bodies.
b) The characteristics of the products or goods to be tested or inspected and the testing or inspection methods are specified in the corresponding standards and technical regulations.
1.3. Processing the conformity assessment results:
Compare the characteristics of the products or goods based on the test or inspection results with the requirements.
1.4. Concluding conformity:
Products or goods are considered conforming if all the indicators of the tested or inspected products or goods meet the specified levels of the corresponding standards and technical regulations.
2. Principles for using Method 8:
Method 8 is used to assess the conformity of products or goods that have strict safety requirements before being circulated or used./.
PHỤ LỤC III
FORMS USED IN STANDARDS CONFORMITY DECLARATION AND TECHNICAL REGULATIONS CONFORMITY DECLARATION
(Issued together with Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology)
1. Quality Control Plan:
Form 1. KHKSCL
28/2012/TT-BKHCN.
2. Standards Conformity Declaration/Technical Regulations Conformity Declaration:
Form 2. CBHC/HQ
28/2012/TT-BKHCN.
3. Notice of Receipt of Standards Conformity Declaration/Technical Regulations Conformity Declaration Dossier:
Form 3. TBTNHS
28/2012/TT-BKHCN.
4. Report on the Reception of Standards Conformity Declaration/Technical Regulations Conformity Declaration Dossiers:
Form 4. BCTNHS
28/2012/TT-BKHCN.
5. Standards Conformity/Technical Regulations Conformity Assessment Report:
Form 5. BCĐG
28/2012/TT-BKHCN.
Form 1. KHKSCL
28/2012/TT-BKHCN
KẾ HOẠCH KIỂM SOÁT CHẤT LƯỢNG
Sản phẩm/hàng hóa/dịch vụ/quá trình/môi trường:……………………………………..
| Các quá trình sản xuất cụ thể | Kế hoạch kiểm soát chất lượng | ||||||
| Các chỉ tiêu giám sát/kiểm soát | Tiêu chuẩn/quy chuẩn kỹ thuật | Tần suất lấy mẫu/cỡ mẫu | Thiết bị thử nghiệm/kiểm tra | Phương pháp thử/kiểm tra | Biểu ghi chép | Ghi chú | |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
|
|
|
|
|
|
|
|
|
|
| …………, ngày ……tháng …….năm………. |
Form 2. CBHC/HQ 1
28/2012/TT-BKHCN
CỘNG HÒA XÃ HỘI CHỦ NGHĨA VIỆT NAM
Độc lập - Tự do - Hạnh phúc
---------------
BẢN CÔNG BỐ HỢP CHUẨN/HỢP QUY
Số…………………
Tên tổ chức, cá nhân:...................................................................................................
Địa chỉ:........................................................................................................................
Điện thoại:………………………………Fax:......................................................................
E-mail:.........................................................................................................................
CÔNG BỐ:
Sản phẩm, hàng hóa, quá trình, dịch vụ, môi trường (tên gọi, kiểu, loại, nhãn hiệu, đặc trưng kỹ thuật,….)
...................................................................................................................................
...................................................................................................................................
Phù hợp với tiêu chuẩn/quy chuẩn kỹ thuật (số hiệu, ký hiệu, tên gọi)
...................................................................................................................................
...................................................................................................................................
Thông tin bổ sung (căn cứ công bố hợp chuẩn/hợp quy, phương thức đánh giá sự phù hợp….)
...................................................................................................................................
...................................................................................................................................
Loại hình đánh giá:
+ Tổ chức chứng nhận đánh giá (bên thứ ba): Tên tổ chức chứng nhận/tổ chức chứng nhận được chỉ định, số giấy chứng nhận, ngày cấp giấy chứng nhận;
...................................................................................................................................
...................................................................................................................................
+ Tự đánh giá (bên thứ nhất): Ngày lãnh đạo tổ chức, cá nhân ký xác nhận Báo cáo tự đánh giá.
...................................................................................................................................
...................................................................................................................................
....(Tên tổ chức, cá nhân).... cam kết và chịu trách nhiệm về tính phù hợp của ……. (sản phẩm, hàng hóa, quá trình, dịch vụ, môi trường) do mình sản xuất, kinh doanh, bảo quản, vận chuyển, sử dụng, khai thác.
|
| ……….. , ngày ……tháng ……năm …… |
_____________
1 Đoạn từ : "Loại hình đánh giá: + Tổ chức chứng nhận đánh giá (bên thứ ba): Tên tổ chức chứng nhận/tổ chức chứng nhận được chỉ định, số giấy chứng nhận, ngày cấp giấy chứng nhận + Tự đánh giá (bên thứ nhất): Ngày lãnh đạo tổ chức, cá nhân ký xác nhận Báo cáo tự đánh giá...." được bổ sung theo quy định tại khoản 7 Điều 1 Thông tư số 02/2017/TT-BKHCN sửa đổi, bổ sung một số điều của Thông tư số 28/2012/TT-BKHCN ngày 12 tháng 12 năm 2012 của Bộ trưởng Bộ Khoa học và Công nghệ quy định về công bố hợp chuẩn, công bố hợp quy và phương thức đánh giá sự phù hợp với tiêu chuẩn, quy chuẩn kỹ thuật, có hiệu lực kể từ ngày 15 tháng 5 năm 2017.
Form 3. TBTNHS
28/2012/TT-BKHCN
| TÊN CƠ QUAN CHỦ QUẢN | CỘNG HÒA XÃ HỘI CHỦ NGHĨA VIỆT NAM |
| Số: …….../TB-…… | ………, ngày … tháng …. năm ….. |
THÔNG BÁO
TIẾP NHẬN HỒ SƠ CÔNG BỐ HỢP CHUẨN/HỢP QUY
……. (Tên cơ quan tiếp nhận công bố) …… xác nhận đã tiếp nhận hồ sơ công bố hợp chuẩn/hợp quy số …. ngày …….. tháng …… năm …….. của:…………………………… (tên tổ chức, cá nhân) ………………………………………………………………………………
địa chỉ tổ chức, cá nhân: …………………………………………………………………………
cho sản phẩm, hàng hóa, quá trình, dịch vụ, môi trường (tên gọi, kiểu, loại, nhãn hiệu, đặc trưng kỹ thuật...): …………………………………………………………………….
phù hợp tiêu chuẩn (số hiệu, ký hiệu, tên gọi tiêu chuẩn)/quy chuẩn kỹ thuật (số hiệu, ký hiệu, tên gọi quy chuẩn kỹ thuật) và có giá trị đến ngày ….. tháng …… năm ……. (hoặc ghi: có giá trị 3 năm kể từ ngày …… tháng ……. năm ….).
Thông báo này ghi nhận sự cam kết của tổ chức, cá nhân. Thông báo này không có giá trị chứng nhận cho sản phẩm, hàng hóa, quá trình, dịch vụ, môi trường phù hợp với tiêu chuẩn/quy chuẩn kỹ thuật tương ứng.
(Tên tổ chức, cá nhân) …… phải hoàn toàn chịu trách nhiệm về tính phù hợp của sản phẩm, hàng hóa, quá trình, dịch vụ, môi trường do mình sản xuất, kinh doanh, bảo quản, vận chuyển, sử dụng, khai thác.
|
Nơi nhận: | Đại diện có thẩm quyền của |
1. Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, is based on the following:
“Pursuant to the Law on Standards and Technical Regulations dated June 29, 2006;
Pursuant to the Law on Product and Goods Quality dated November 21, 2007;
Pursuant to Decree No. 127/2007/ND-CP dated August 1, 2007, of the Government detailing the implementation of a number of articles of the Law on Standards and Technical Regulations;
Pursuant to Decree No. 132/2008/ND-CP dated December 31, 2008, of the Government detailing the implementation of a number of articles of the Law on Product and Goods Quality;
Pursuant to Decree No. 20/2013/ND-CP dated February 26, 2013, of the Government defining the functions, tasks, powers, and organizational structure of the Ministry of Science and Technology;
At the request of the Director General of the Directorate for Standards, Metrology, and Quality and the Director of the Legal Department;
The Minister of Science and Technology promulgates the Circular amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations.”
2. This clause is amended according to the provisions of Clause 1, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
3. This clause is amended according to the provisions of Clause 1, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
4. This clause is added according to the provisions of Clause 1, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
5. This article is amended and supplemented according to the provisions of Clause 2, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
6. This article is amended and supplemented according to the provisions of Clause 3, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
7. This article is amended and supplemented according to the provisions of Clause 4, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
8. This clause is amended according to the provisions of Clause 5, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
9. This point is amended according to the provisions of Point b, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
10. This point is added according to the provisions of Point a, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
11. This point is amended and supplemented according to the provisions of Point c, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
12. This point is amended according to the provisions of Point d, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
13. This point is amended according to the provisions of Point đ, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
14. This point is amended according to the provisions of Point đ, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
15. This point is amended according to the provisions of Point e, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
16. This point is amended according to the provisions of Point e, Clause 6, Article 1 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017.
17. Article 2 of Circular No. 02/2017/TT-BKHCN amending and supplementing a number of articles of Circular No. 28/2012/TT-BKHCN dated December 12, 2012, by the Minister of Science and Technology regulating standards conformity declaration, technical regulations conformity declaration, and methods of conformity assessment with standards and technical regulations, effective from May 15, 2017, is as follows:
“Article 2. Implementation Provisions
1. This Circular takes effect from May 15, 2017.
2. The Notices of Receipt of Standards Conformity Declaration and Technical Regulations Conformity Declaration dossiers continue to be valid until the expiration date of the issued Notices.”















